Abstract
Background: Serious complications during SARS-CoV2 infection were reported in onco-haematological patients; in addition, COVID-19 can also indirectly force the interruption or delay of cancer treatment. Early administration of oral antivirals [molnupiravir (MOL) and nirmatrelvir/ritonavir (NIR/r)] or monoclonal antibody (Sotrovimab) may reduce hospitalization and death risk. However, data in hematological patients are lacking.
Methods: Outpatients diagnosed with onco-hematologic malignancy presenting COVID-19 were treated with oral antivirals. Hospitalization and lung failure rate, overall mortality, and safety were analyzed.
Results: Overall, 83 outpatients were prospectively enrolled in the study [median (q1-q3) age of 61 (48 - 72) years; males in 52% of cases]. All subjects were affected by B.1.1.529 (omicron) SARS-CoV2 variant. Notably, 75 (90%) were fully vaccinated against SARS-CoV2.
A total of 33 (40%) was affected by HL/NHL, 21 (25%) by AML/MDS, 14 (17%) by CLL, 15 (18%) by other malignancies, including 4 ALL, 9 MM, and 2 CML.
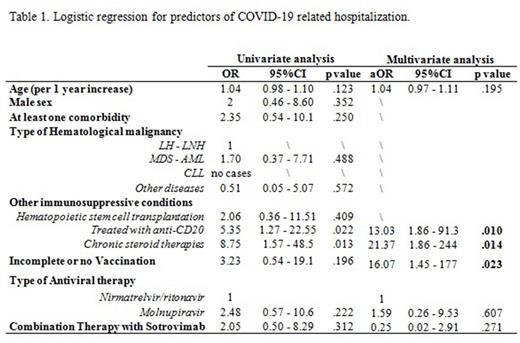
At the time of COVID-19, 11 (13%) were hematopoietic-stem-cell transplant recipients, 19 (23%) were treated with anti-CD20, 7 (8%) were under chronic steroid therapy.
After multidisciplinary evaluation, 44 (53%) and 39 (47%) were treated with NIR/r and MOL, respectively; in addition, 14 (17%) patients underwent a combination therapy with Sotrovimab. Median (q1-q3) time from diagnosis of COVID-19 to therapy was of 1 (1-2) days. No grade 3-4 adverse events related to antiviral or Sotrovimab were reported.
Overall, 7 (8%) patients were hospitalized for COVID-19 despite therapy. By performing a univariate and multivariate logistic regression, predictors of hospitalization were: recent administration of anti-CD20 (aOR=13.03, 95%CI=1.86-91.3), chronic steroid therapy (aOR=21.37, 95%CI=1.86-244), incomplete/lack of vaccination (aOR=16.07, 95%CI=1.45-177).
Interestingly, the subgroup of patients affected by HL/NHL and CLL presented a longer viral shedding, if compared with AML/MDS and other malignancies groups (14 vs 14 vs 8 vs 8 days respectively, p=.031); similarly, persisting COVID-19 symptoms (>21 days) was significantly more frequent in HL/NHL group vs others (18% vs 0% in other groups, p=.020).
Conclusions: The early administration of oral antivirals may be safe in onco-hematologic outpatients and could reduce the risk of hospitalization and death due to SARS-COV2 infection. Nevertheless, some groups of patients are still at higher risk and need further attention.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal